INTRODUCTION
Plants are natural reservoir of medicinal
agents that are almost free of side effects normally caused by synthetic
chemicals (Fennell et al., 2004), Medicinal plants play an important role in the health of
people living in rural and urban societies (Focho et al., 2009). A number of compounds
have been isolated from natural sources and many of these compounds were based
on the uses of the agents in traditional medicine (Rizvi
et al., 2009). The overuse of
synthetic drugs with impurities resulting in a higher incidence of adverse drug
reactions has motivated mankind to go back to nature for safe remedies (WHO
1999). The World Health Organization (WHO) estimates that 75 - 80% of the
population of developing countries currently use medicinal plants as remedies because
of better cultural acceptability, better compatibility with the human body (Kamboj 2000; Yandav and Dixit, 2008). A single plant may be used for
the treatment of various disease conditions depending on the community. Several
ailments including fever, asthma, constipation, esophageal
cancer, and hypertension have been treated with traditional medicinal plants
(Cousins and Huffman, 2002; Saganuwan, 2010). In Africa, the use of the medicinal
plant has been the unique health care for 4000 years, long before the advent of
western medicine (Silverthorn et al., 2010). Currently, there is a renewed interest in
drugs of natural origin simple, because they are green medicine and green
medicine offer safe, effective treatment, with minimal or no side effect, easily
available, cheap cost and are in great demand in the developed World health
care.
Chemotherapy
with effective antibiotic drugs remains the main method to control bacterial
infections in the absence of a suitable vaccine treatment (WHO 2011).
Unfortunately, the bacterial infections have developed resistance against many
of the antibacterial drugs couple with the fact that the newly produced
effective anti-bacteria drugs are expensive, especially for most poor Nigerians
to afford. These have resulted in therapy failure, hence, the need to continue
to explore indigenous knowledge of traditional medicine and therapeutic
potential of plants through pharmacological research, bioprospecting
and drug discovery.
Nelsonia canescens have been used for a long time in diverse contexts, i.e. as an
ornamental plant, antioxidant (Sawadogo et al., 2006) antibacterial,
anti-inflammatory, analgesic, purgative, and antispasmodic (Focho
et al., 2009). Nelsonia canescens (Lam.) Spreng
(Family Acanthaceae)
commonly called blue pussy leaf with the synonyms Justicia brunelloides (Lam) (Mahias
et al., 2007), is found
growing in secondary wet evergreen forests, savannah forests and open disturbed
habitats, especially in moist areas along roadsides, trails, and as a weed in
agricultural land (McDade, 2012). The genus Nelsonia is usually
treated in the subfamily Nelsonioideae within the Acanthaceae (McDade, 2008).
This study is
aimed at evaluating the quantitative phytochemical constituents and
antimicrobial activity of aqueous leaves extract of blue pussy leaf Nelsonia canescens.
MATERIALS AND METHODS
Plant Collection and identification.
The leaf of Nelsonia canescens was collected in January 2017
from Banana plantations of the Doko community in Lavun Local Government Area of Niger State, Nigeria. The
community is located at a (Latitude (80 N and Longitude 50 E
(Adekun,1978).The leaves were identified and authenticated in the Department of
Biology Science, Kebbi State University of Science
and Technology Aliero and assigned a voucher number:
(V.N.148 A).
Bacterial Strains
The bacteria use for the biological test
include the gram-positive Staphylococcus aureus and
Streptococcus pyogenes, gram-negative are Pseudomonas aeruginosa,
E. Coli and Salmonella typhi
Plant processing and
Extraction.
Fresh leaves
of Nelsonia canescens were shade-dried for 21 days at room
temperature (27 – 29.5°C). The dried leaves were pounded using mortar and
pestle into powdered form. One hundred and fifty grams of the plant powder was
macerated with 3 L of distilled water with continued shaking agitation at room
temperature. The extract was filtered every 24 hours using muslin cloth which
was followed by a further filtration using Whatman
filter paper No. 1 having a pore size of 0.7 um. The filtrate was further
concentrated using the rotary evaporator at 450C to give a dark
semi-solid extract. The extracts obtained were stored in an air-tight amber
bottle and refrigerated at 40C prior to use (Mann, 2007).
Quantitative Phytochemical Estimation of the Aqueous leaves extract of
Nelsonia canescens
Determination of Total Phenol
Zero-point five (0.5) mL (1mg/mL) was oxidized
with 2.5 mL of 10% Folin Ciocalteau’s
reagent (v/v) and neutralized by 2mL of 7.5% sodium carbonate. The reaction
mixture was incubated for 40 minutes at 45°C and the absorbance was taken at
765nm using the double beam Shimadzu UV spectrophotometer, UV-1800. The total
phenolic content was subsequently calculated using Gallic acid as standard (Singleton et al., 1999)
Determination of Total Flavonoid
Total flavonoid was determined using aluminum chloride colorimetric method (Chang et al., 2002). Quercetin
was used to establish the calibration curve. Exactly 0.5mL of the diluted
sample was added into a test tube containing 1.5ml of methanol,
0.1ml of 10% AlCl3 solution and 0.1ml sodium acetate (NaCH3COO־) were added, followed by
2.8ml of distilled water. After incubation at room temperature for 30min, the
absorbance of the reaction mixture was measured at 415nm with a double beam
Shimadzu UV spectrophotometer, UV-1800. The amount of 10% AlCl3 was
substituted by the same amount of distilled water in blank.
Determination of Total Alkaloids
Zero-point five (0.5g) of the sample was
dissolved in 96% ethanol - 20% H2SO4 (1:1). 1ml of the
filtrate was added to 5mL of 60% tetraoxosulphate
(VI) and allowed to stand for 5min. 5mL of 0.5% formaldehyde was added and
allowed to stand for 3h. The reading was taken at the absorbance of 565nm. The
extinction coefficient (E296, ethanol {ETOH} = 15136M־¹cm־¹) of vincristine was used as reference alkaloid
(Oloyede, 2005)
Determination of Saponins
Zero-point five (0.5g) of the sample was added
to 20ml of 1NHCl and was boiled for 4h. After cooling it was filtered and 50ml
of petroleum ether was added to the filtrate for the ether layer and evaporated
to dryness. 5ml of acetone ethanol was added to the residue and 0.4mls of each
was taken into 3 different test tubes. 6ml of ferrous sulphate reagent was
added into them followed by 2ml of concentrated H2SO4. It
was thoroughly mixed after 10min and the absorbance was taken at 490nm.
Standard saponin was used to establish the
calibration curve (Oloyed, 2005).
Determination of Tannin
Zero-point two (0.2g) of the sample was
measured into a 50ml beaker. 20mL of 50% methanol was added and covered with parafilm and placed in a water bath at 77-80ᴼC for
1hr., it was shaken thoroughly to ensure a uniform mixture. The extract was
quantitatively filtered using a double-layered Whatman
No.41 filter paper into a 100ml volumetric flask, 20ml water added, 2.5ml Folin-Denis reagent and 10ml of 17% Na2CO3
were added and mixed properly. The mixture was made up to mark with water,
mixed well and allowed to stand for 20min for the development of a bluish-green
colouration. The absorbances of the tannic acid
standard solutions, as well as samples, were read after colour development on a
UV-spectrophotometer model UV-1800, at a wavelength of 760nm (Emmanuel et al., 2014).
Antimicrobial
activity of Nelsonia canescens
The antimicrobial activity of Nelsonia canescens was
carried out by the cork borer method in which a 6mm sterile cork
borer which was sterilized by flame and used to bore four wells in the
solidified nutrient agar plates aseptically (Oyeleke et al., 2008). The nutrient agar plates
were then inoculated with 3 hours incubated suspension of the bacteria isolates
using a sterile swab stick which was seeded evenly on the surface of the agar
plate. Each well was filled with the crude extract of Nelsonia canescens
samples of different concentrations mg/ml i.e. 40mg/ml, 30mg/ml, 20mg/ml
and 100mg/ml Ampiclox control and these were repeated
for each test organism. The plates were then incubated at 370C for
24 hours. The inhibition zone around each well was measured.
Determination of Minimum Inhibitory Concentration and Minimum
Bactericidal Concentration
The tube dilution method was used to determine
the MIC and MBC of the active extract. A two-fold and seven series (40, 20, 10,
5, 2.5, 1.25 and 0.625 mg/ml) dilutions of each extract were prepared in
Nutrient broth. Zero-point one millilitre (0.1 ml) of each of the standardized
test organisms (0.5 McFarland turbidity standard) was
added to each dilution. One control tube was also prepared which contain only
sterile medium without the test organisms or the extract after which all tubes
were incubated in a water bath with a shaker at 37°C for 24 hours. The culture
was incubated at 37°C for 24 hours. The lowest concentration of the subcultured medium without visible growth was recorded as
the minimum bactericidal concentration (Cheesebrough,
2006).
Statistical analysis
Results were expressed as mean ± Standard Error of Mean (SEM). All the
data were analyzed by one-way ANOVA and differences
between the means were assessed with the Ducan
Multiple comparison test. Differences were considered significant at p<
0.05. All the analyses were carried out using Statistical Package for Social
Science (SPSS) version 20 (USA).
RESULTS AND DISCUSSION
Table 1. Quantitative phytochemical analysis of
aqueous leaf extract of N. canescens
|
Phytochemicals
|
Amount (mg/100g)
|
|
Alkaloids
|
107.04±0.62
|
|
Total phenols
|
144.60±1.76
|
|
Flavonoids
|
72.50±4.17
|
|
Saponins
|
330.00±0.00
|
|
Tannins
|
42.17±1.17
|
Table 2. Mean zones of inhibition of aqueous leaf extract N. canescens
|
Bacteria
|
20 mg/ml
|
30 mg/ml
|
40mg/ml
|
*Ampiclox(1mg/ml)
|
|
S. aureus
|
18.00±0.00a
|
19.50±0.50a
|
19.50±0.50a
|
19.50±2.50a
|
|
P.aeruginosa
|
18.00±1.00b
|
19.00±0.00ab
|
20.50±0.50c
|
0.00±0.00a
|
|
E. coli
|
20.50±0.50b
|
19.50±0.50b
|
24.50±0.50c
|
16.00±1.00a
|
|
S. pyogenes
|
17.50±0.50a
|
17.00±1.00a
|
19.00±0.00a
|
28.00±1.00b
|
|
S. typhi
|
14.50±0.50a
|
18.00±1.00b
|
20.50±0.50b
|
13.00±1.00a
|
|
|
|
|
|
|
Values are expressed in mean ± standard error
of mean.
Values with
the same superscript on the same row have no significant difference at
p<0.05
Specification for Ampiclox:
≤ 12 mm (resistant), 13-17 mm (intermediate), ≥ 18 mm (susceptible)
(CLSI, 2012).
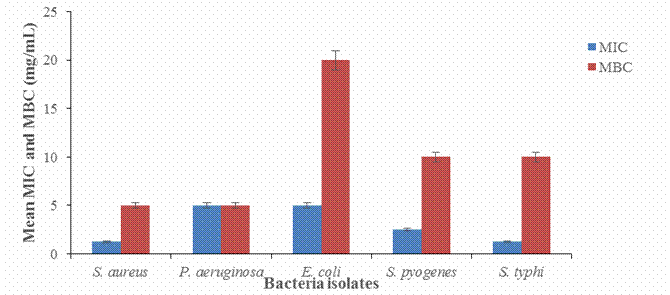
Figure 1. MIC and MBC of aqueous
leaf extract of N. canescens
DISCUSSION
The results
in Table 1 indicated high amount of alkaloids (107.04±0.62mg/g), total Phenol
(144.60±1.76 mg/g), flavonoids 72.50±4.17mg/g, saponins
(330.00±0.00mg/g) and tannins (42.17±1.17mg/g). These quantitative
phytochemicals in aqueous leaves extract of Nelsonia canescens in (Table 1) is responsible
for preventing infectious diseases and therefore could explain their use
traditionally for the treatment of wide ranges of illness including treatment
of pain, chickenpox, measles, Inflammations, constipation and gastric ulcer (Owoyele, et al.,
2005; Acharya et
al., 2012). In Africa, Nelsonia canescens is used to reduce fever and as an analgesic
in a wide range of conditions including colds, flu, and viral infections
(PROTA, 2014). The presence of a variety of phytochemicals in the present study
gives the indication that the plant's extracts could be used for curative
activity against pathogens and therefore could explain their use traditionally
for the treatment of a wide array of illness including malaria (Anaduaka et al.,
2013). The presence of alkaloids in plant extracts is also used for a wide
range of pharmacological activities including antimalarial, antiasthma,
anticancer (Kittakoop et al., 2014).
Results
from table 2 and figure 1, reveals the antibacterial activity of the aqueous
leaf extract of Nelsonia canescens which indicate how active the
plant extract is to the test organisms, the result when compared with the
standard drug showed statistical significance (P < 0.05), even though the
plant extract is still in the crude form. The specification for susceptibility
for the standard drug is the zones of inhibition from 18 mm above, the plant
extract was observed also to be concentration-dependent such that, increase in
the concentration gives a direct increase in the inhibition zones which cut
across all the test organisms. Escherichia coli was the most
susceptible organism having the highest mean zone of inhibition of 20.50±0.50
at 20 mg/ml with the MIC at 5.0 mg/ml and MBC of 20 mg/ml, followed by the Staphylococcus aurus
with mean zone of inhibition of 18.00±0.00 at 20 mg/ml, this was also evident in the MIC at 1.25 mg/ml and MBC of 5.0
mg/ml. The plant crude extract was active against Gram-negative bacteria;
Pseudomonas aeruginosa, Escherichia coli, and Salmonella, typhi.
Gram-positive bacteria, Staphylococcus aurus
and Streptococcus
pyogenes had wide zones of inhibition. This work
is in agreement with the finding of (Wayne, 2002). The minimum inhibitory
concentrations (MIC) of the crude extract against the test organisms generally
were low suggesting that the plant extract will be highly effective against the
test organisms if developed as drugs for the treatment of infections. Therefore
it could be concluded that aqueous leaf extract of Nelsonia canescens exert its antibacterial
(sensitive bacteria) activities against the selected microorganisms and can,
therefore, be used to develop drugs that can be employed for the treatment of
infections caused by these organisms. Also, the activity of the plant extract
is dose-dependent as an increase in activity was observed when the
concentration was increased. Since secondary metabolites are usually known to
be more active against gram-positive bacterium and mostly inactive against
gram-negative bacterium (Agbafor et al., 2011).
CONCLUSION
The present study has shown that the aqueous
leaf extract of Nelsonia canescens
possesses phytochemical constituents. This antibacterial potential is
probably due partly to the high contents of alkaloids, total phenolics, flavonoids, saponins,
and tannins. These scientific data allows us to justify the traditional use of Nelsonia canescens
for treatment of pain, reduce fever, inflammation, constipation and gastric
ulcer. Further studies will involve the identification of the active
ingredients and isolations of the functional groups present in the plant
extract.
REFERENCES
Acharya RN, Padiya RH,
Patel ED, Harisha CR, Shukla
VJ, Chauhan MG. Pharmacognostic
(2012) evaluation of Nelsonia canescens (Lam.)
Spreng (Acanthaceae) root. Pharmacogn J.
28:45–8.
Adekun
O. (1978). Atlas of Federal Republic of Nigeria, Ibadan, Onibonoje
pres and Book industries Ltd., Pp12.
Agbafor, K.N., E.I. Akubugwo,
M.E. Ogbashi, P.M. Ajah and
C.C. Ukwandu, (2011). Chemical and antimicrobial properties of leaf
extracts of Zapoteca portoricensis. Res.
J. Med. Plant, 5: 605-612.l
Anaduaka E.G, Ogugua V.N, Egba SI, Apeh V.O (2013) Investigation of some important of
phytochemical, nutritional properties and toxicological potentials of ethanol
extracts of New boldialaevis leaf and Stem. Afr. J, Biotecnol. 12(40):5846-5354.
Chang, C., Yang, M., Wen, H. and Chern, J.
(2002). Estimation
of total flavonoid content in Propolis by two Complementary colorimetric
methods. Journal of Food Drug Analysis, 10: 178-182.
Cheesebrough M. (2006). District laboratory practices in tropical countries. Cambridge University Press, Edinburgh, United Kingdom. 382–407.
Cousins D, Huffman MA
(2002). Medicinal properties in the diet of gorillas:
an ethno-pharmacolgical evaluation. African study
Monographs, 23(2):65-89.
Emmanuel, E. O., Helmina, O. and Egwim, C. E. (2014). Phytochemical Constituents
of Seeds of Ripe and Unripe Blighia Sapida (K. Koenig) and Physicochemical Properties of the
Seed Oil. International Journal of Pharmaceutical Science Invention, 3
(9): 31-40.
Fennell, C.W, Lindsey,K.L., McGaw, L, J., Sparg, S.G., Stafford, G.I., Elgorashi,
E.E., Grace, O.M. and van Staden, J. (2004).
Assessing African medicinal plants for efficacy and safety: Pharmacology
screening and toxicity. Journal of Ethnopharmacology.94:205-217.
Focho, D.A, Ndam, W.T.
and Fogne, B.A (2009). “Medicinal plants of Aguambu-Bamumbu
in the Lebialem highlands, southwest province of
Cameroon” African Journal of Pharmacology,
3(1): 001-013.
Kamboj,
V.P. (2000). Herbal medicine. Current Science. 78(1):35-39.
Kittakoop P, Mihidol C, Ruchirawat
S (2014). Alkaloids as important scaffolds in therapeutic
drugs for the treatments of cancer, tuberculosis and smoking cessation. Curr. Top
Med. Chem. 14(2):239-255.
Mahias,
SN, Ilyas, N and Musa, KY (2007) “Phytochemical
constituents of some medicinal plants use amongst the Takkad
people of Southern Kaduna-Nigeria” ChemClass Journal
CSN Zaria, 4: 70-75
Mann, A. (2007). Survey of ethnomedicine for the treatment
of tuberculosis; Chemistry perspective. Ayanwola
printing works, Minna Niger State, Nigeria. P 117.
McDade
L. A. (2008). Toward a comprehensive understanding of phylogenetic relationship
among lineages of Acanthancea S. L(Lamiales). American Journal of Botany, 95(9): 1136-1152.
McDade,
L. A. (2012). Phylogenic placement, delimitation, and relationships among
genera of the enigmatic Nelsonsonia (Lamiales: Acanthaceae). Taxon,
61(3):63 – 76.
Oloyed,
O. I. (2005). Chemical profile of unripe pulp of Carica pagaya. Pakistan
Journal of Nutrition, 4: 379-381.
Owoyele, VB, Oloriegbe, YY, Balogun,
EA, Soladoye, AO (2005) “Analgesic and
anti-inflammatory properties of Nelsonia canescens leaf extract” Journal of Ethnopharmacology.
99: 153-156
Oyeleke, S.B and Manga, B.S (2008). Essentials of Laboratory
Practical in Microbiology, Tobest publisher Minna. Pp.20-70.
PROTA, (2014). PROTA4U web database. Grubben G. J. H, Denton O.
A, eds. Wageningen, Netherlands: Plant Resources of Tropical
Africa http://www.prota4u.org/search.asp
Rizvi, M.M.A, Irshad, M.,
Hassadi G.E. and Younis,
S.B. (2009). Bioefficacies of
cassia fistula; An Indian Labrum (Review). African journal of pharmacy and
pharmacology, 3(6): 287- 301.
Saganuwan A. S (2010). Some medicinal plants of Arabian
Peninsula. African. Journal of Microbial., 4(9): 766-788.
Sawadogo, WR, Meda, R, Lamien, CE, Kiendrebeogo, M, Guissou, IP and Nacoulma, OG (2006). Phenolic content and
antioxidant activity of six Acanthaceae from Burkina
Faso. Journal of Biological
Sciences, 6(2): 249-252.
Silverthorn, D. U (2010). “Fisiologia
umana. Un approccio integrato” quinta edizione, pp. 354-358
Singleton, V. L., Orthofer, R. and Lamuela-Raventos, R. M. (1999). Analysis of total phenols and
other oxidation substrates and antioxidants by means of Folin- Ciocalteu
reagent. Methods in Enzymology, 299: 152-178
Wayne P. (2002). Clinical
and laboratory standard institute. Performance
standard for antimicrobial disc susceptibility testing. National
Committee for Clinica
W.H.O. (1999). WHO Monographs on Selected Medicinal Plants. 1: 1-295.
World Health Organization (WHO) (2011). World malaria report, 2011.
World Health Organization, Geneva,Switzerland.
Available
Yandav, N.P. and Dixit, V.K. (2008). Recent approaches in herbal drug
standardization. International
journal of Integrative Biology. 2(3); 195-203.

